
BLACK LIGHT COLOR SPECTRUM SKIN
UVA rays have a longer wavelength that can penetrate the middle layer of your skin (the dermis)Ī: UVC radiation is the highest energy portion of the UV radiation spectrum.UVB rays have a short wavelength that reaches the outer layer of your skin (the epidermis).
BLACK LIGHT COLOR SPECTRUM SERIES
Wavelength describes the distance between the peaks in a series of waves. Like all forms of light on the EM spectrum, UV radiation is classified by wavelength. So, most of the UV rays you come in contact with are UVA with a small amount of UVB. While UVA and UVB rays are transmitted through the atmosphere, all UVC and some UVB rays are absorbed by the Earth’s ozone layer. UVA rays have the longest wavelengths, followed by UVB, and UVC rays which have the shortest wavelengths. The most common form of UV radiation is sunlight, which produces three main types of UV rays: More Information on the Electromagnetic Spectrum Q: What are the different types of UV radiation? At the top of the spectrum, gamma rays have photons with very high energies and short wavelengths with peaks that are close together. The photons of microwaves have higher energies, followed by infrared waves, UV rays, and X-rays. These ranges describe the activity level, or how energetic the photons are, and the size of the wavelength in each category.įor example, at the bottom of the spectrum radio waves have photons with low energies, so their wavelengths are long with peaks that are far apart. The EM spectrum is divided into categories defined by a range of numbers. All EM radiation (also called EM energy) is made up of minute packets of energy or 'particles,' called photons, which travel in a wave-like pattern and move at the speed of light. More Information on UV Radiation Q: How is radiation classified on the electromagnetic spectrum?Įlectromagnetic radiation is all around us, though we can only see some of it.
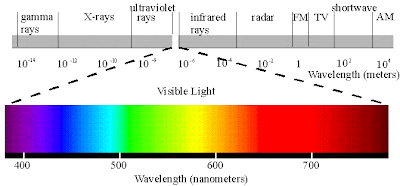
UV radiation is the portion of the EM spectrum between X-rays and visible light. Radio waves that transmit sound from a radio station’s tower to your stereo, or between cell phones microwaves, like those that heat your food in a microwave oven visible light that is emitted from the lights in your home and X-rays like those used in hospital X-ray machines to capture images of the bones inside your body, are all forms of EM energy. UV radiation is only one type of EM energy you may be familiar with. UV radiation is only one form of radiation and it is measured on a scientific scale called the electromagnetic (EM) spectrum. Does where I live affect the amount of UV radiation I am exposed to?Īll radiation is a form of energy, most of which is invisible to the human eye.Are there health benefits of exposure to UV radiation?.What effect does UV radiation have on my body?.What are the risks associated with using some UVC lamps?.What are the risks of exposure to UVC radiation?.What are the different types of UV radiation?.How is radiation classified on the electromagnetic spectrum?.I think this is when white light is used that you get an Absorption Spectra. All the colors of the Absorption Spectra do make it kind of confusing. And these are being absorbed (with emphasis on blue). Actually, if you just burned hydrogen and looked at its spectra, you would get the Emission Spectra and not the Absorption Spectra, and this Emission Spectra would only show the bunch of blue lines, one purple line, and one red line. All the other colors shown are just part of the natural light being shown down on the element. This is the color that will be the opposite of the flame color on the color wheel. Remember, always look at the color area on the rainbow that is blacked out the most. So if blue is being absorbed, the opposite color would be transmitted and this color is orange. However, there are MORE dark lines in the blue region. If you look at the lines for hydrogen blue, purple, and red are being absorbed. Therefore, all the other colors would be absorbed. (This would be orange.) The element hydrogen turns orange when being burned and this color is transmitted to us. This means that if there is a big dark band where blue would be, then the opposite color to blue on the color wheel is being transmitted. You are supposed to look at the dark areas of the absorption spectra and those dark areas indicate that the color which would be there is being absorbed. I think both the absorption and emission lines are showing which colors are being absorbed.


 0 kommentar(er)
0 kommentar(er)
